Of2 lewis structure molecular geometry hybridization polarity and
Table of Contents
Table of Contents
Molecular orbital diagrams are an essential tool used to display the arrangement of electrons in molecules. Drawing molecular orbital diagrams for polyatomic molecules can be a daunting task for many students. However, with the right approach and understanding, one can master the art of drawing molecular orbital diagrams for polyatomic molecules.
How to Draw Molecular Orbital Diagrams for Polyatomic Molecules
Understanding how to draw molecular orbital diagrams for polyatomic molecules can be challenging. It is essential to note that the process involved is not as complicated as it may seem. The primary challenge that students encounter when drawing molecular orbital diagrams for polyatomic molecules is determining the molecular and electronic geometries. This can be confusing, especially when dealing with larger molecules.
To draw molecular orbital diagrams for polyatomic molecules, you will need to follow a step-by-step approach. The first step involves determining the electronic configuration of the molecule. This is important as it enables you to identify the molecular orbitals that are occupied and the ones that are not. The second step involves filling the molecular orbitals with electrons, starting from the lowest energy to the highest. Lastly, you will need to draw the molecular orbital diagram, which involves plotting the molecular orbitals on the y-axis and the atomic nuclei on the x-axis.
To summarize, when drawing molecular orbital diagrams for polyatomic molecules, you need to determine the electronic configuration of the molecule, fill the molecular orbitals with electrons, and draw the molecular orbital diagram. These steps will enable you to understand the electron arrangement in the molecule.
Targeting the Process of Drawing Molecular Orbital Diagrams for Polyatomic Molecules
When I was in college, I struggled with drawing molecular orbital diagrams for polyatomic molecules. I would get lost in the technical jargon and could not figure out the right approach. However, with time and practice, I learned how to draw molecular orbital diagrams for polyatomic molecules with ease. If you are struggling with drawing these diagrams, don’t worry, below is a step-by-step guide on how to draw molecular orbital diagrams for polyatomic molecules.
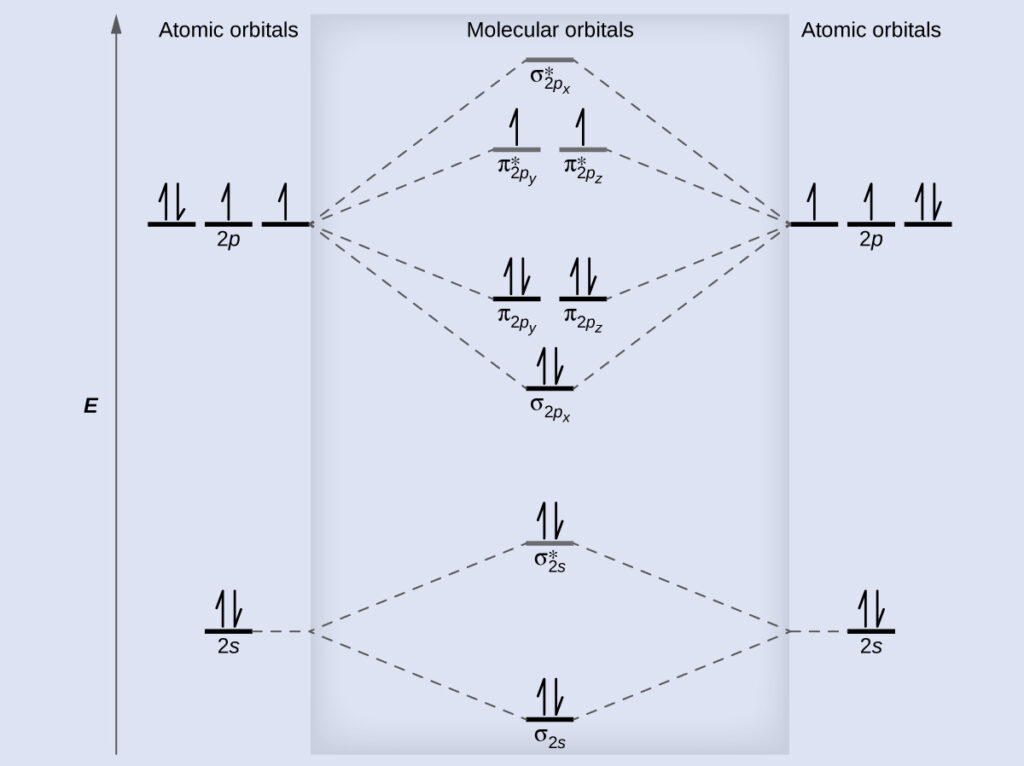 The first step is to determine the electronic configuration of the molecule. This will enable you to identify the molecular orbitals that are occupied and the ones that are not. The second step involves filling the molecular orbitals with electrons, starting from the lowest energy to the highest. The last step involves plotting the molecular orbitals on the y-axis and the atomic nuclei on the x-axis to create a molecular orbital diagram.
The first step is to determine the electronic configuration of the molecule. This will enable you to identify the molecular orbitals that are occupied and the ones that are not. The second step involves filling the molecular orbitals with electrons, starting from the lowest energy to the highest. The last step involves plotting the molecular orbitals on the y-axis and the atomic nuclei on the x-axis to create a molecular orbital diagram.
Targeting the Common Mistakes Students Make when Drawing Molecular Orbital Diagrams for Polyatomic Molecules
Drawing molecular orbital diagrams for polyatomic molecules can be tricky, and there are some common mistakes that students make. One such mistake is failing to identify the electronic configuration of the molecule. Failing to do this can result in incorrect electron arrangements in the molecular orbital diagram. Another common mistake is filling the molecular orbitals with electrons haphazardly, which can result in an incorrect diagram. To avoid these mistakes, always take your time and follow the steps carefully.
Targeting Understanding the Different Types of Molecular Orbitals
There are three types of molecular orbitals, namely bonding orbitals, anti-bonding orbitals, and non-bonding orbitals. Bonding orbitals are lower in energy and result from constructive interference, while anti-bonding orbitals are higher in energy and result from destructive interference. Non-bonding orbitals are not involved in bonding and are generally found on lone atoms or atoms with no other electrons to bond with.
 #### Targeting the Significance of Molecular Orbital Theory
#### Targeting the Significance of Molecular Orbital Theory
Molecular orbital theory is essential as it helps us understand the chemical and physical properties of molecules. For instance, it helps us understand why some molecules are more stable than others and why some molecules are more reactive than others. Additionally, it helps us predict the geometry and polarity of a molecule, which is essential in understanding its behavior.
Targeting an Example of Drawing Molecular Orbital Diagrams for Polyatomic Molecules
Let’s consider the example of drawing a molecular orbital diagram for water. The electronic configuration of oxygen is 1s2 2s2 2p4, while that of hydrogen is 1s1. Therefore, the electronic configuration of water would be (1s2)(2s2)(2px2)(2py1)(2pz1).
 From the electronic configuration of water, we can see that the two 1s electrons of hydrogen occupy the two lowest-energy MOs, while the four 2s and 2p electrons of oxygen occupy the four higher-energy MOs. This arrangement results in the formation of two bonding MOs and two anti-bonding MOs. The two MOs that contain the hydrogen 1s electrons are the bonding MOs, while the two MOs with no electrons are the anti-bonding MOs.
From the electronic configuration of water, we can see that the two 1s electrons of hydrogen occupy the two lowest-energy MOs, while the four 2s and 2p electrons of oxygen occupy the four higher-energy MOs. This arrangement results in the formation of two bonding MOs and two anti-bonding MOs. The two MOs that contain the hydrogen 1s electrons are the bonding MOs, while the two MOs with no electrons are the anti-bonding MOs.
Question and Answer
Q: Why is it essential to identify the electronic configuration of a molecule when drawing molecular orbital diagrams for polyatomic molecules?
A: Identifying the electronic configuration of a molecule is essential as it enables you to determine the molecular orbitals that are occupied and the ones that are not. This is important when filling the molecular orbitals with electrons and drawing the molecular orbital diagrams.
Q: What are the different types of molecular orbitals?
A: The three types of molecular orbitals are bonding orbitals, anti-bonding orbitals, and non-bonding orbitals.
Q: Why is molecular orbital theory important?
A: Molecular orbital theory is important as it helps us understand the chemical and physical properties of molecules. Additionally, it helps us predict the geometry and polarity of a molecule, which is essential in understanding its behavior.
Q: What are some common mistakes that students make when drawing molecular orbital diagrams for polyatomic molecules?
A: Common mistakes include failing to identify the electronic configuration of the molecule, filling the molecular orbitals with electrons haphazardly, and failing to plot the molecular orbitals on the y-axis and the atomic nuclei on the x-axis.
Conclusion of how to draw molecular orbital diagrams for polyatomic molecules
Drawing molecular orbital diagrams for polyatomic molecules may seem daunting, but it is not as complicated as it may seem. With the right approach and understanding, anyone can master the art of drawing molecular orbital diagrams for polyatomic molecules. Remember to identify the electronic configuration of the molecule, fill the molecular orbitals with electrons, and plot the molecular orbitals on the y-axis and the atomic nuclei on the x-axis.
Gallery
How To Draw Molecular Orbital Diagrams For Polyatomic Molecules

Photo Credit by: bing.com /
OF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, And

Photo Credit by: bing.com / orbital molecular nh3 orbitals diatomic molecules of2 bonding atomic delocalized libretexts chem techiescientist o2 hybridization conclusion valence explain homonuclear electrons
How To Draw Molecular Orbital Diagrams For Polyatomic Molecules

Photo Credit by: bing.com /
Mathematics - Origins Of Molecular Orbital Diagrams? - History Of

Photo Credit by: bing.com / orbital molecular diagrams does origins molecules chemistry mathematics description electrons questions
Use The Molecular Orbital Diagram Shown To Determine Which Of The

Photo Credit by: bing.com / orbital molecular diagrams determine simplified