Covalent lewis dot bonds draw bond weebly
Table of Contents
Table of Contents
Are you struggling with drawing lewis structures for covalent bonds? Do the diagrams and lines seem confusing and overwhelming? Fear not! In this article, we will go over the basics of how to draw lewis structures for covalent bonds, step-by-step.
When it comes to chemistry, drawing lewis structures for covalent bonds can be a pain point for many students. It involves understanding the sharing of electrons between atoms, and applying specific rules to represent these bonds in a diagram. However, with a little practice and patience, anyone can become proficient in drawing lewis structures.
To get started, you first need to identify the atoms in the molecule you want to draw a lewis structure for. Determine the total number of valence electrons that each atom has, and the total number of valence electrons for the entire molecule. From there, you can begin to arrange the atoms in a way that makes sense, and connect them with single bonds. Then, place the remaining electrons around each atom according to the octet rule, until all valence electrons have been accounted for.
Overall, the key to drawing lewis structures for covalent bonds is practice and familiarity with the rules. Remember to follow the steps outlined above, and you’ll be well on your way to mastering lewis structures for covalent bonds.
Steps to drawing lewis structures for covalent bonds
When I first learned about drawing lewis structures, I found it helpful to break down the process into specific steps. Here are the basic steps you can follow to draw a lewis structure for a covalent bond:
Step 1: Identify the atoms in the molecule and determine their valence electrons.
 Step 2: Determine the total number of valence electrons for the entire molecule.
Step 2: Determine the total number of valence electrons for the entire molecule.
Step 3: Draw the skeletal structure of the molecule, showing the single bonds between the atoms.
Step 4: Place the remaining electrons around each atom according to the octet rule.
 Common mistakes to avoid
Common mistakes to avoid
One common mistake students make when drawing lewis structures for covalent bonds is forgetting to account for all valence electrons. Another mistake is placing too many or too few electrons on an atom, violating the octet rule. It’s important to double-check your work to ensure that all electrons have been accounted for and that each atom follows the octet rule.
Additional Tips
It can be helpful to practice drawing lewis structures for different types of molecules to become more familiar with the process. Additionally, don’t be afraid to seek out additional resources, such as online tutorials or study guides.
Practice problem
Draw the lewis structure for H2O.
Question and Answer
Q: What is a covalent bond?
A: A covalent bond is a chemical bond formed by the sharing of electrons between atoms.
Q: What is the octet rule?
A: The octet rule states that atoms tend to gain, lose, or share electrons in order to have a full outer shell of eight electrons.
Q: How do you determine the total number of valence electrons for a molecule?
A: To determine the total number of valence electrons for a molecule, you add together the valence electrons for each individual atom.
Q: Can you have a covalent bond between two atoms of the same element?
A: Yes, covalent bonds can occur between atoms of the same element, such as in diatomic molecules like O2 and N2.
Conclusion of how to draw lewis structures for covalent bonds
Drawing lewis structures for covalent bonds can seem complicated at first, but with practice and patience, anyone can master the process. Remember to follow the steps outlined above and double-check your work to avoid common mistakes. By understanding the basics of drawing lewis structures, you’ll be able to analyze molecules and better understand their properties and behavior.
Gallery
PPT - Lewis Dot Structures Of Covalent Compounds PowerPoint
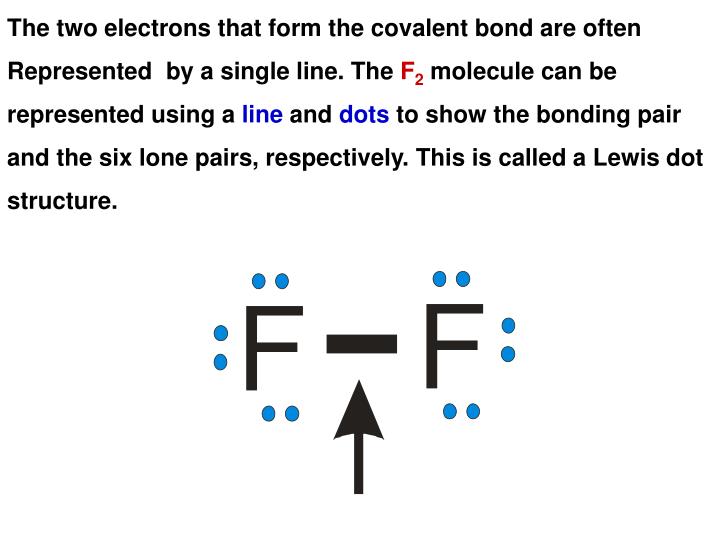
Photo Credit by: bing.com / covalent compounds represented molecule
Covalent Bond

Photo Credit by: bing.com / covalent lewis dot bonds draw bond weebly
Lewis Dot Diagram For Carbon - Wiring Diagram
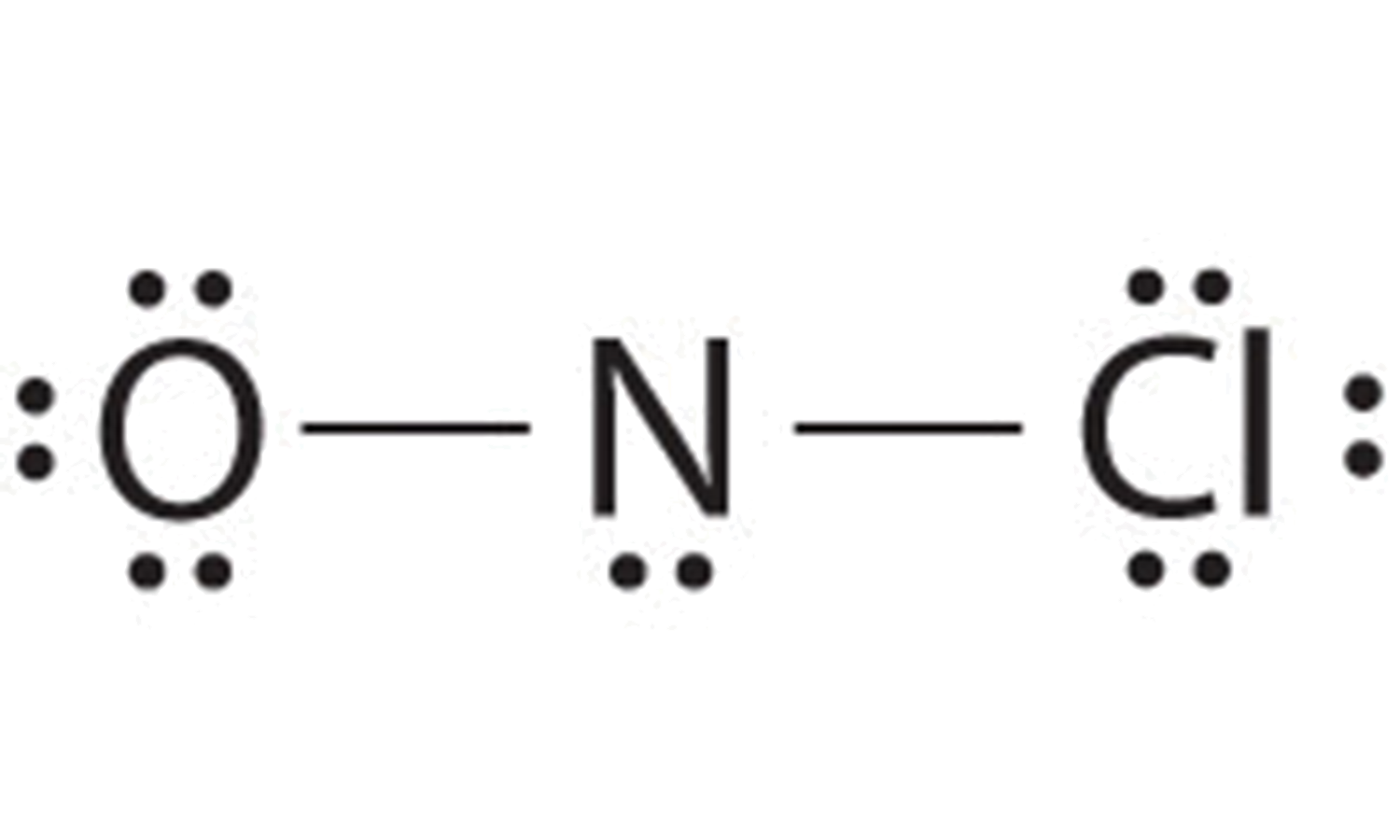
Photo Credit by: bing.com / lewis dot diagram structures carbon chemistry drawing libretexts chem
Chemistry Notes

Photo Credit by: bing.com /
[DIAGRAM] Chemical Bonds Lewis Dot Diagram FULL Version HD Quality Dot
![[DIAGRAM] Chemical Bonds Lewis Dot Diagram FULL Version HD Quality Dot [DIAGRAM] Chemical Bonds Lewis Dot Diagram FULL Version HD Quality Dot](https://i.ytimg.com/vi/3VpgPYPuFs4/maxresdefault.jpg)
Photo Credit by: bing.com / covalent compounds bonds ionic





